Phenotypes associated with this allele
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Ikbkbtm2Mka mutation
(0 available);
any
Ikbkb mutation
(54 available)
Tg(Ckmm-cre)1Lrsn mutation
(0 available)
|
|
|
muscle
|
|
• under long-term differentiation conditions without medium replenishment, myotubes are 48% less atrophic compared to similarly treated wild-type mouse embryonic fibroblasts
|
|
|
• the number of intermediate fibers is increased compared to in wild-type mice
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Igf2rtm1Rlj mutation
(0 available);
any
Igf2r mutation
(98 available)
Tg(Ckmm-cre)1Lrsn mutation
(0 available)
|
|
|
normal phenotype
cellular
|
|
• the paternally-inherited Igf2r allele is normally silenced through genomic imprinting so embryos inheriting the floxed Igf2r allele maternally are functionally muscle-specific homozygous knockouts
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Fxntm2.1Mkn mutation
(0 available);
any
Fxn mutation
(40 available)
Fxntm2Mkn mutation
(0 available);
any
Fxn mutation
(40 available)
Tg(Ckmm-cre)1Lrsn mutation
(0 available)
|
|
|
mortality/aging
|
|
• mutant mice die at 76 10 days (10-12 weeks)
(J:75420)
• antioxidant idebenone delays death by 1 week
(J:90401)
|
cardiovascular system
|
|
• at 10 weeks, mutant mice exhibit sparse atrophied myofibrils which are pushed to the periphery, as well as disrupted myofibrils at the positions of intercalated discs
(J:75420)
• earlier, at 4 weeks, mutant hearts display abnormal accumulation of lipid droplets and few degenerating fibers; by 5-6 weeks, a gradual reduction in lipid droplets, swollen mitochondria, and disorganized cardiac muscle fibers are observed
(J:90401)
|
|
|
• at 10 weeks, mutant mice display many disorganized mitochondria with central tubular cristae, and electron-dense iron deposits in the matrix of mitochondria in cardiac muscle; only rare swollen mitochondria are observed
|
|
|
• by 10 weeks, mutant mice show myocardial degeneration with cytoplasmic vacuolization in myocytes, indicating necrosis
|
|
|
• at 7 weeks, mutant mice display normal or slightly increased iron content in cardiac mitochondria relative to wild-type mice (791 108 ng iron/mg protein vs 749 35 ng iron/mg protein, respectively)
(J:75420)
• however, at 10 weeks, cardiac mitochondrial iron is significantly increased (1,049 205 ng iron/mg protein vs 579 65 ng iron/mg protein)
(J:75420)
• cardiac intramitochondrial iron concentration significantly increases between 8-9 weeks to reach ~2-fold the normal iron content by death
(J:90401)
|
|
|
• starting at 5 weeks, mutant hearts show a significantly increased ventricular septum wall thickness
|
|
|
• at 7 weeks, mutant mice exhibit a progressively increasing heart to body weight ratio, reaching 18.6 5 mg/g vs 5.6 0.4 mg/g for wild-type littermates at death
• no evidence in heart size difference is noted at 2-3 weeks of age
|
|
|
• starting at 5 weeks, mutant hearts exhibit a significantly increased left ventricular mass
|
|
|
• by 10 weeks, mutant mice display notable thickening of the left ventricular wall
|
|
|
• starting at 5 weeks, mutant hearts show significantly increased left ventricular posterior wall thickness
|
|
|
• starting at 5 weeks, mutant hearts display a significant increase in left ventricle diastolic and systolic diameters
|
|
|
• by 10 weeks, mutant mice show post-necrotic fibrosis in cardiac muscle
|
|
|
• mutant mice display an initial cardiac hypertrophy which develops into a dilated cardiomyopathy without skeletal muscle involvement
(J:75420)
• at 7 weeks, hypertrophic cardiomyopathy is rapidly associated with significant geometric remodeling
(J:90401)
• antioxidant idebenone delays the cardiac disease onset, progression and death by 1 week, but fails to correct the Fe-S enzyme deficiency and has no effect on the status of lipid peroxidation (oxidative stress)
(J:90401)
|
|
|
• at 8 weeks of age, mutant mice show a 67% reduction of resting cardiac output, associated with saturated hypertrophy
|
|
|
• at 5 and 7 weeks of age, mutant mice show a 24% and 66% decrease of the shortening fraction, respectively
|
|
|
• during tribromoethanol anesthesia, most 9-wk-old mutants display a prolonged PR interval that progresses from a first- to a third-degree (complete) atrioventricular block with severe bradycardia
|
cellular
|
|
• by 10 weeks, mutant mice show myocardial degeneration with cytoplasmic vacuolization in myocytes, indicating necrosis
|
|
|
• at 12 weeks, mutant hearts display mitochondrial degeneration; excessive accumulation of abnormal mitochondria displaces fibers to the periphery
|
|
|
• at 10 weeks, mutant mice display many disorganized mitochondria with central tubular cristae, and electron-dense iron deposits in the matrix of mitochondria in cardiac muscle; only rare swollen mitochondria are observed
|
|
|
• at 7 and 10 weeks, mutants display a marked succinate dehydrogenase deficiency (complex II) in cardiac muscle, with no significant difference in cytochrome c oxidase activity (complex IV)
(J:75420)
• at 7 and 10 weeks, mutants show a 77-87% complex II deficiency and a 70-74% aconitase deficiency in cardiac muscle; complex I (NADH dehydrogenase) and III (cytochrome c reductase) activities are also significantly reduced
(J:75420)
• Fe-S deficiency in cardiac muscle occurs very early in disease pathology (at 4 weeks), with 50% residual activity, whereas mitochondrial iron accumulation occurs at 4-5 weeks after the onset of heart pathology and Fe-S deficit and serves as a marker of disease end stage
(J:90401)
• from 7 weeks onward, reduced Fe-S enzyme activities are associated with lower levels of oxidative stress markers (oxidized proteins) in cardiac muscle, with reduced lipid peroxide levels noted at 9 weeks
(J:90401)
|
|
|
• at 10 weeks, mutant mice show increased mitochondrial proliferation in cardiac muscle
|
homeostasis/metabolism
growth/size/body
|
|
• at 7 weeks, mutant mice exhibit a progressively increasing heart to body weight ratio, reaching 18.6 5 mg/g vs 5.6 0.4 mg/g for wild-type littermates at death
• no evidence in heart size difference is noted at 2-3 weeks of age
|
|
|
• mutant mice begin to lose weight at ~7 weeks of age, reaching a 29% weight reduction at death
|
behavior/neurological
|
|
• mutant mice develop signs of fatigue prior to death
|
muscle
|
|
• at 10 weeks, mutant mice exhibit sparse atrophied myofibrils which are pushed to the periphery, as well as disrupted myofibrils at the positions of intercalated discs
(J:75420)
• earlier, at 4 weeks, mutant hearts display abnormal accumulation of lipid droplets and few degenerating fibers; by 5-6 weeks, a gradual reduction in lipid droplets, swollen mitochondria, and disorganized cardiac muscle fibers are observed
(J:90401)
|
|
|
• at 10 weeks, mutant mice display many disorganized mitochondria with central tubular cristae, and electron-dense iron deposits in the matrix of mitochondria in cardiac muscle; only rare swollen mitochondria are observed
|
|
|
• by 10 weeks, mutant mice show myocardial degeneration with cytoplasmic vacuolization in myocytes, indicating necrosis
|
|
|
• mutant mice display an initial cardiac hypertrophy which develops into a dilated cardiomyopathy without skeletal muscle involvement
(J:75420)
• at 7 weeks, hypertrophic cardiomyopathy is rapidly associated with significant geometric remodeling
(J:90401)
• antioxidant idebenone delays the cardiac disease onset, progression and death by 1 week, but fails to correct the Fe-S enzyme deficiency and has no effect on the status of lipid peroxidation (oxidative stress)
(J:90401)
|
|
|
• at 5 and 7 weeks of age, mutant mice show a 24% and 66% decrease of the shortening fraction, respectively
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Tfamtm1Lrsn mutation
(1 available);
any
Tfam mutation
(11 available)
Tg(Ckmm-cre)1Lrsn mutation
(0 available)
|
|
|
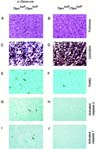
Heart histology of Tfamtm1Lrsn/Tfamtm1Lrsn Tg(Ckmm-cre)1Lrsn/0 mice
mortality/aging
|
|
• die at 2-4 weeks of age
|
growth/size/body
|
|
• cessation of weight gain from P10 onwards
|
cardiovascular system
|
|
• ECG changes under isofluorane anesthesia
|
|
|
• decrease in peak aortic blood flow velocity under isofluorane anesthesia
|
|
|
• mutants exhibit a significant increase in apoptosis of cardiomyocytes
• however, no evidence of fibrosis, necrosis, or inflammatory cell infiltration is seen in the hearts
|
homeostasis/metabolism
behavior/neurological
|
|
• decreased spontaneous movement from P10 onwards
|
muscle
|
|
• mutants exhibit a significant increase in apoptosis of cardiomyocytes
• however, no evidence of fibrosis, necrosis, or inflammatory cell infiltration is seen in the hearts
|
cellular
|
|
• mutants exhibit a significant increase in apoptosis of cardiomyocytes
• however, no evidence of fibrosis, necrosis, or inflammatory cell infiltration is seen in the hearts
|
|
|
• mosaic respiratory chain deficiency in the myocardium
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Ilktm1Star mutation
(1 available);
any
Ilk mutation
(18 available)
Tg(Ckmm-cre)1Lrsn mutation
(0 available)
|
|
|
mortality/aging
|
|
• all mice die suddenly starting at about 6 weeks of age, between 6 and 12 weeks of age and with a median age of death of 2 months
• mice often die during mating and during attempted surgical procedures and hearts show evidence of stress at the molecular level, indicating increased cardiac physiological stress
|
cardiovascular system
|
|
• disaggregation of adjacent cardiomyocytes within heart tissue
• however, mice show no evidence of skeletal muscle defects
|
|
|
• hearts are grossly enlarged, with a 2-fold increase in the heart-to-body mass ratio
|
|
|
• dilated left ventricular chambers
|
|
|
• fibrosis in left ventricle and accumulation of interstitial fibrotic tissue in hearts
|
|
|
• mice exhibit enlarged hearts and impaired contraction of hearts leading to heart failure by 6-12 weeks of age
|
 |
• ejection fraction is greatly reduced, indicating impaired pumping capacity of the heart
|
 |
• echocardiography indicates an increase in end diastolic and end systolic areas and reduced ejection fraction
|
|
|
• mice exhibit labored breathing, lack of physical strength, disorientation, problems with balance, and hunched, withdrawn behavior before death, indicating heart failure
|
muscle
|
|
• mice exhibit enlarged hearts and impaired contraction of hearts leading to heart failure by 6-12 weeks of age
|
 |
• ejection fraction is greatly reduced, indicating impaired pumping capacity of the heart
|
growth/size/body
|
|
• hearts are grossly enlarged, with a 2-fold increase in the heart-to-body mass ratio
|
cellular
|
|
• fibrosis in left ventricle and accumulation of interstitial fibrotic tissue in hearts
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Erbb2tm1Klee mutation
(0 available);
any
Erbb2 mutation
(59 available)
Tg(Ckmm-cre)1Lrsn mutation
(0 available)
|
|
|
cardiovascular system
|
|
• increase in the numbers of mitochondria and vacuoles in cardiomyoctyes, however cytoskeletal ultrastructure is unchanged
|
|
|
• increase in apoptosis in the ventricles
|
|
|
• decrease in LV septal thickness
|
|
|
• decrease in LV posterior ventricular wall thickness
|
|
|
• all mutants develop dilated cardiomyopathy by 6 weeks of age
• exhbiit ventricular enlargement of both the left and right cardiac chambers and a marked increase in heart:body weight ratio
|
|
|
• decrease in fractional shortening, velocity of circumferential fiber shortening and a reduction of the maximum first derivative of left ventricle pressure, indicating depressed myocardium contractility, however no differences in heart rate or left ventricle end-diastolic pressure
|
|
|
• reduction in left ventricle dP/dtmin indicates impaired left ventricle relaxation
|
muscle
|
|
• increase in the numbers of mitochondria and vacuoles in cardiomyoctyes, however cytoskeletal ultrastructure is unchanged
|
|
|
• all mutants develop dilated cardiomyopathy by 6 weeks of age
• exhbiit ventricular enlargement of both the left and right cardiac chambers and a marked increase in heart:body weight ratio
|
|
|
• decrease in fractional shortening, velocity of circumferential fiber shortening and a reduction of the maximum first derivative of left ventricle pressure, indicating depressed myocardium contractility, however no differences in heart rate or left ventricle end-diastolic pressure
|
|
|
• reduction in left ventricle dP/dtmin indicates impaired left ventricle relaxation
|



 Analysis Tools
Analysis Tools
