Phenotypes associated with this allele
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Smarcb1tm2Sho mutation
(0 available);
any
Smarcb1 mutation
(22 available)
Tg(GFAP-cre)#Gtm mutation
(0 available)
Trp53tm1Brn mutation
(18 available);
any
Trp53 mutation
(232 available)
|
|
|
nervous system
|
|
• lesions in the white matter of the corpus callosum and cingulum
|
neoplasm
|
N |
• no neoplastic growth is seen in the brain
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Smarcb1tm2Sho mutation
(0 available);
any
Smarcb1 mutation
(22 available)
Tg(GFAP-cre)#Gtm mutation
(0 available)
Trp53tm1Brn mutation
(18 available);
any
Trp53 mutation
(232 available)
|
|
|
behavior/neurological
|
|
• excessive scratching behavior resulting in dermatitis around the neck, earlobes, and the flank region
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Smarcb1tm2Sho mutation
(0 available);
any
Smarcb1 mutation
(22 available)
Tg(GFAP-cre)#Gtm mutation
(0 available)
Trp53tm1Brn mutation
(18 available);
any
Trp53 mutation
(232 available)
|
|
|
behavior/neurological
|
|
• excessive scratching behavior resulting in dermatitis around the neck, earlobes, and the flank region
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Smarcb1tm2Sho mutation
(0 available);
any
Smarcb1 mutation
(22 available)
Tg(GFAP-cre)#Gtm mutation
(0 available)
Trp53tm1Brn mutation
(18 available);
any
Trp53 mutation
(232 available)
|
|
|
behavior/neurological
|
|
• many mice appear lethargic with hind limb paralysis
|
|
|
• severe scratching behavior
|
|
|
• mice exhibit intermittent episodes of tail extension and dystonic posturing of the body, frequently in response to handling
|
|
|
• motor coordination problems become evident from around 3 weeks of age
|
|
|
• abnormal posture of the hind limbs
|
|
|
• mice exhibit tumbling repeatedly during attempted locomotion; mice frequently fall over and repeatedly lift and replace the same paw in various positions during each stride
|
|
|
• mice exhibit a non-uniform gait, with uneven stride length and width, dragging of the hindpaws and an inability to keep the hindquarters upr
|
|
|
• many mice appear lethargic with hind limb paralysis
|
|
|
• mice exhibit clear seizures or intermittent electrographic seizures associated either with forelimb clonus, evolving into body jerking, and wild running or arrest of activity during the ictal EEG discharges
|
cellular
|
|
• altered granule neuron migration, with some granule neurons failing to migrate out of the external germinal layer, others remaining trapped in the molecular layer
• altered cerebellar granule neuron migration is already seen at 3 weeks of age
|
growth/size/body
|
|
• body weight is reduced by about 40%
|
mortality/aging
|
|
• mice require water gel for survival
|
muscle
|
|
• mice exhibit intermittent episodes of tail extension and dystonic posturing of the body, frequently in response to handling
|
neoplasm
|
|
• mice develop brain tumors starting at around 1 month of age
• mice that die suddenly exhibit presence of high-grade, aggressive tumors that infiltrate and obliterate the surrounding cerebellar folia
• tumors appear to arise from the cerebellum
• tumors show hallmarks of atypical teratoid/rhabdoid tumor of the central nervous system
|
nervous system
|
|
• mice exhibit clear seizures or intermittent electrographic seizures associated either with forelimb clonus, evolving into body jerking, and wild running or arrest of activity during the ictal EEG discharges
|
|
|
• altered granule neuron migration, with some granule neurons failing to migrate out of the external germinal layer, others remaining trapped in the molecular layer
• altered cerebellar granule neuron migration is already seen at 3 weeks of age
|
|
|
• mice develop brain tumors starting at around 1 month of age
• mice that die suddenly exhibit presence of high-grade, aggressive tumors that infiltrate and obliterate the surrounding cerebellar folia
• tumors appear to arise from the cerebellum
• tumors show hallmarks of atypical teratoid/rhabdoid tumor of the central nervous system
|
|
|
• defects in white matter with complete loss of tissue in regions of white matter over time
• white matter fiber tracks are reduced
• loss of white matter is already seen at 3 weeks of age
|
|
|
• defects in the corpus callosum
• lesions in the corpus callosum are already seen at 3 weeks of age
|
|
|
• defects in the hippocampus
|
|
|
• reduced cerebral cortex is already seen at 3 weeks of age
|
|
|
• defects in the cytoarchitecture of the cerebellum
• progressive loss of cells and neuronal projections in the cerebellum
|
|
|
• aberrant external germinal layer
|
|
|
• the boundary between the internal granule layer and the molecular layer remains diffuse
|
|
|
• loss of oligodendrocytes with age
|
|
|
• high level of gliosis in the fiber tracks and in the molecular layer and in the remnants of the external germinal layer
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Itgavtm2.1Hyn mutation
(0 available);
any
Itgav mutation
(53 available)
Itgavtm2Hyn mutation
(2 available);
any
Itgav mutation
(53 available)
Tg(GFAP-cre)#Gtm mutation
(0 available)
|
|
|
cardiovascular system
|
|
• at E18 and P5, mice exhibit microhemorrhage in the cerebellum, mid-brain, and cerebral cortex unlike in control mice
|
|
|
• at E18 and P5, mice exhibit microhemorrhage in the cerebellum unlike in control mice
|
|
|
• at E18 and P5, mice exhibit microhemorrhage in the cerebral cortex unlike in control mice
• between P7 and P14, mice exhibit progressive reduction in cerebral microhemorrhage
• however, adult mice exhibit no hemorrhage or edema in the brain
|
nervous system
|
|
• at E18 and P5, mice exhibit microhemorrhage in the cerebellum, mid-brain, and cerebral cortex unlike in control mice
|
|
|
• at E18 and P5, mice exhibit microhemorrhage in the cerebellum unlike in control mice
|
|
|
• at E18 and P5, mice exhibit microhemorrhage in the cerebral cortex unlike in control mice
• between P7 and P14, mice exhibit progressive reduction in cerebral microhemorrhage
• however, adult mice exhibit no hemorrhage or edema in the brain
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Smarcb1tm2Sho mutation
(0 available);
any
Smarcb1 mutation
(22 available)
Tg(GFAP-cre)#Gtm mutation
(0 available)
|
|
|
nervous system
|
|
• variable, mild defect in granule neuron migration
|
|
|
• brain lesions that are prominent in the white matter of the corpus callosum with vacuolization, spongy changes, cystic-like breakdown and destruction of tissue
• extent of damage is variable and progresses with age
|
|
|
• posterior portion of the corpus callosum is more affected than the anterior portion
|
|
|
• disorganization of the Purkinje cell layer
|
cellular
|
|
• variable, mild defect in granule neuron migration
|
neoplasm
|
N |
• mice do not develop brain tumors up to more than 1 year of age
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Tg(GFAP-cre)#Gtm mutation
(0 available)
Tsc1tm1Djk mutation
(2 available);
any
Tsc1 mutation
(69 available)
|
|
|
mortality/aging
|
|
• mice die by 6 to 10 weeks of age
|
nervous system
|
|
• by 6 to 10 weeks of age
|
behavior/neurological
|
|
• by 6 to 10 weeks of age
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Gt(ROSA)26Sortm2(tTA,CMV*1-KIAA1549/BRAF,-EGFP)Gtm mutation
(0 available);
any
Gt(ROSA)26Sor mutation
(942 available)
Tg(GFAP-cre)#Gtm mutation
(0 available)
|
|
|
nervous system
|
N |
• mice exhibit normal GFAP+ and S100B+ astrocytes cell numbers
|
hematopoietic system
|
|
• at 9 months of age, mutants have significantly more microglia than controls; microglia are in the optic gliomas
|
immune system
|
|
• at 9 months of age, mutants have significantly more microglia than controls; microglia are in the optic gliomas
|
nervous system
|
|
• at 9 months of age, mutants have significantly more microglia than controls; microglia are in the optic gliomas
|
|
|
• mice develop optic gliomas in the optic chiasm/nerves
• optic gliomas appear as gross thickenings and enlargements of the pre-chiasmic nerve at 9 months of age
|
|
|
• irregularly shaped and thickened astrocytes are seen in the pre-chiasmatic optic nerves
|
|
|
• retinal ganglion cell loss in the middle and peripheral region
|
|
|
• optic gliomas form in the pre-chiasmatic optic nerve/optic chiasm, with increased vascularization to the area, abnormally shaped and thickened astrocytes, increase in microglia, and axonal and myelin degeneration
• mice exhibit abnormal optic nerve axon organization
|
|
|
• the optic chiasm/nerves at 9 months of age appear as gross fusiform enlargements that result in optic gliomas
• increase in vascularization in the pre-chiasmatic optic nerve and optic chiasm (and in the optic glioma)
|
|
|
• axonal and myelin degeneration are seen in the optic nerve
|
|
|
• increase in neoplastic astrocyte proliferation in the optic glioma
|
|
|
• disruption of the myelin is seen in the optic nerve, including degeneration and hypermyelination
|
|
|
• hypermyelination is seen in the optic nerve
|
neoplasm
|
|
• mice develop optic gliomas in the optic chiasm/nerves
• optic gliomas appear as gross thickenings and enlargements of the pre-chiasmic nerve at 9 months of age
|
vision/eye
|
|
• retinal ganglion cell loss in the middle and peripheral region
|
|
|
• optic gliomas form in the pre-chiasmatic optic nerve/optic chiasm, with increased vascularization to the area, abnormally shaped and thickened astrocytes, increase in microglia, and axonal and myelin degeneration
• mice exhibit abnormal optic nerve axon organization
|
|
|
• the optic chiasm/nerves at 9 months of age appear as gross fusiform enlargements that result in optic gliomas
• increase in vascularization in the pre-chiasmatic optic nerve and optic chiasm (and in the optic glioma)
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Nf1tm1Fcr mutation
(3 available);
any
Nf1 mutation
(157 available)
Tg(GFAP-cre)#Gtm mutation
(0 available)
Tsc1tm1Djk mutation
(2 available);
any
Tsc1 mutation
(69 available)
|
|
|
mortality/aging
|
|
• most mice die before 3 months of age
|
neoplasm
|
N |
• mice do not develop gliomas
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Nf1tm1Fcr mutation
(3 available);
any
Nf1 mutation
(157 available)
Tg(GFAP-cre)#Gtm mutation
(0 available)
|
|
|
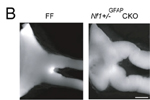
Nf1tm1Fcr/Nf1+ Tg(GFAP-cre)#Gtm/0 mice develop prechiasmatic optic nerve and chiasmal gliomas
nervous system
|
|
• cell proliferation in the optic nerve is increased compared to in wild-type mice
|
|
|
• kinking of the prechiasmatic optic nerves
|
neoplasm
vision/eye
|
|
• kinking of the prechiasmatic optic nerves
|
cellular
|
|
• cell proliferation in the optic nerve is increased compared to in wild-type mice
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Gt(ROSA)26Sortm1(tTA,CMV*1-Rheb,-EGFP)Gtm mutation
(0 available);
any
Gt(ROSA)26Sor mutation
(942 available)
Tg(GFAP-cre)#Gtm mutation
(0 available)
|
|
|
nervous system
|
N |
• mice exhibit normal brain weight
• mice do not develop seizures
|
|
|
• astrocytes exhibit increased mTor signaling-dependent proliferation compared to in control cells
|
growth/size/body
|
N |
• mice exhibit normal body weight
|



