Phenotypes associated with this allele
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Tbx1tm1Bem mutation
(1 available);
any
Tbx1 mutation
(34 available)
|
|
|
cardiovascular system
|
|
• 2 of 15 had a right sided aortic arch
|
|
|
• 15 of 15 had persistent truncus arteriosis
|
craniofacial
|
|
• 15 of 15 had an overt cleft palate
|
immune system
|
|
• a single lobe thyroid gland was present
|
hematopoietic system
|
|
• a single lobe thyroid gland was present
|
digestive/alimentary system
|
|
• 15 of 15 had an overt cleft palate
|
endocrine/exocrine glands
|
|
• a single lobe thyroid gland was present
|
embryo
muscle
hearing/vestibular/ear
|
|
• lack of defined inner ear structures
|
|
|
• missing middle ear structures
|
growth/size/body
|
|
• 15 of 15 had an overt cleft palate
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Tbx1tm1Bem mutation
(1 available);
any
Tbx1 mutation
(34 available)
|
|
|
cardiovascular system
|
|
• embryos show show a severe, single outflow vessel phenotype at E17.5
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Tbx1tm1Bem mutation
(1 available);
any
Tbx1 mutation
(34 available)
|
|
|
hearing/vestibular/ear
|
|
• the otocyst is hypoplastic with impaired growth detected during placode invagination, around E9.0
• expression of genes normally seen in the anterior portion of the otocyst are suppressed and expression of posterior genes expanded posterolatterally
|
|
|
• identifiable vestibular and auditory sensory organs fail to form and at E13.5 the inner ear consists of 2 ventral chambers one of which appears to derive from the endolymphatic projection and has cuboidal epithelial cells like those in the wild-type endolymphatic duct
|
nervous system
|
|
• at E9.0 - E11 in the otic vesicle, ectopic neurogenesis is more widespread and persistent than in heterozygotes
|
|
|
• at E10 - E10.5 ectopic delamination and duplication of the cochlear ganglion rudiment is seen with the ganglion volume increased to 1.83-fold that of wild-type
• at E13.5 a secondary compound ganglion is seen apposed to and innervating the epithelial posterior pole and the central projection of the ganglion is absent
• at E14.5 the ganglia are necrotic and mostly absent at after E16.5
|
cellular
|
|
• at E9.0 - E11 in the otic vesicle, ectopic neurogenesis is more widespread and persistent than in heterozygotes
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Tbx1tm1Bem mutation
(1 available);
any
Tbx1 mutation
(34 available)
|
|
|
embryo
|
|
• absent in the caudal branchial region
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Tbx1tm1Bem mutation
(1 available);
any
Tbx1 mutation
(34 available)
|
|
|
behavior/neurological
|
|
• mutants exhibit lower levels of spontaneous alternations in the T-maze at 0 and 30 second delays compared to wild-type mice; both wild-type and mutant mice reach a chance level at a 60 second delay
|
|
|
• mutants exhibit a higher degree of thigmotaxis in the inescapable open field than wild-type mice
• however, mutants are indistinguishable in anxiety-related behaviors in the elevated plus maze from wild-type mice
|
|
|
• mutants initially exhibit higher levels of contact with a novel, non-mouse object compared with wild-type mice
|
|
|
• in the T-maze, mutants visit the same arms across trials more often than wild-type mice when mutants show spontaneous alternation (0 second delay) but not when they do not show spontaneous alternation at a 60 second delay, indicating a repetitive behavioral tendency
|
|
|
• mutants exhibit lower levels of active and passive affiliative social interaction at 2 months of age, but no alterations in aggression, olfactory investigation or motor behavior
|
|
|
• mutant pups are impaired in complex patterns of vocalization but not simple vocal patterns
• pups exhibit vocalization less frequently in complex, two-syllable, composite, frequency steps and flat, and for shorter duration in harmonics, two-syllable, composite, and frequency steps
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Tbx1tm1Bem mutation
(1 available);
any
Tbx1 mutation
(34 available)
|
|
|
cardiovascular system
|
|
• 3 of 29 had outflow tract and pharyngeal arch artery abnormalities including 1 with teratology of fallot with pulmonary atresia, 1 with double outlet right ventricle, and 1 with a retroesophageal right subclavian artery
|
hearing/vestibular/ear
|
|
• 10 of 20 had middle ear abnormalities apparent upon dissection including chronic otitis media, infiltration of inflammatory cells, thickening of the middle ear submucosa, thickening of the bony wall of the bulla, middle ear fluid accumulation, hyperplasia of ciliated cells and associated hearing loss
|
|
|
• the average ABR threshold was 46 dB compared to 29 dB in wild-type mice
|
immune system
Allelic
Composition |
Tbx1tm1Bem/Tbx1+
|
|
Genetic
Background |
involves: 129 * C57BL/6 * CD-1 * SJL |
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Tbx1tm1Bem mutation
(1 available);
any
Tbx1 mutation
(34 available)
|
|
|
cardiovascular system
|
|
• about 25% of embryos show cardiovascular defects at E17.5
|
Allelic
Composition |
Tbx1tm1Bem/Tbx1+
|
|
Genetic
Background |
involves: 129/Sv * C57BL/6J * FVB * SJL |
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Tbx1tm1Bem mutation
(1 available);
any
Tbx1 mutation
(34 available)
|
|
|
nervous system
|
|
• at E9.0 - E11, transient ectopic neurogenesis is seen posteroventromedially and later anterodorsolaterally in the otic vesicle
|
cellular
|
|
• at E9.0 - E11, transient ectopic neurogenesis is seen posteroventromedially and later anterodorsolaterally in the otic vesicle
|
Allelic
Composition |
Tbx1tm1Bem/Tbx1+
|
|
Genetic
Background |
involves: 129/Sv * C57BL/6J * SJL |
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Tbx1tm1Bem mutation
(1 available);
any
Tbx1 mutation
(34 available)
|
|
|
cardiovascular system
|
|
• 2 of 14 have an abnormal origin of the right subclavian artery, an additional 3 of 14 have a retroesophageal right subclavian artery (1 of these also has an interupted aortic arch), and 1 of 14 have a right subclavian artery that arises from the pulmonary artery
|
|
|
• 3 of 14 have a retroesophageal right subclavian artery
|
|
|
• 1 of 14 heterozyotes has an abnormally high aortic arch
|
|
|
• 1 of 14 has an interrupted aortic arch
|
craniofacial
|
|
• hypotrophic fourth arch at E10.5
|
embryo
|
|
• hypotrophic fourth arch at E10.5
|
Allelic
Composition |
Eya1tm1Rilm/Eya1+
Tbx1tm1Bem/Tbx1+
|
|
Genetic
Background |
involves: 129 * C57BL/6 * CD-1 * SJL |
|
|
|
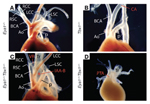
Cardiovascular defects in mice with mutations of one or both copies of Eya1tm1Rilm and Tbx1tm1Bem
cardiovascular system
|
|
• about 73% of embryos show cardiovascular defects at E17.5
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Six1tm1Mair mutation
(0 available);
any
Six1 mutation
(18 available)
Tbx1tm1Bem mutation
(1 available);
any
Tbx1 mutation
(34 available)
|
|
|
cardiovascular system
|
|
• about 63% of embryos show cardiovascular defects at E17.5
|

Cardiovascular defects in mice with mutations of one or both copies of Eya1tm1Rilm and Tbx1tm1Bem
cardiovascular system
|
|
• 100% of embryos display a single outflow vessel
|
Allelic
Composition |
Pitx2tm2Sac/Pitx2+
Tbx1tm1Bem/Tbx1+
|
|
Genetic
Background |
involves: 129S1/Sv * 129X1/SvJ * C57BL/6J * SJL |
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Pitx2tm2Sac mutation
(0 available);
any
Pitx2 mutation
(38 available)
Tbx1tm1Bem mutation
(1 available);
any
Tbx1 mutation
(34 available)
|
|
|
mortality/aging
|
|
• become cyanotic immediately after birth and die
|
cardiovascular system
|
|
• stenosis of the pulmonary trunk
|
|
|
• exhibit severe cardiac defects with incomplete penetrance (about 60%) at E15.5, E18.5 and in newborns
|
|
|
• enlarged atrioventricular canal at E10.5
|
|
|
• occasionally see malformation of the coronary vessels
|
|
|
• occasionally see mispositioning of the aorta
|
|
|
• some show the aorta and the pulmonary artery arising from the right ventricle
|
|
|
• abnormal drainage of the pulmonary vein into a common instead of the left atrium
|
|
|
• atrioventricular valve defects
|
|
|
• hearts are malformed, however they are properly patterned in the atrioventricular canal and around the inner curvature
|
|
|
• reduced ventricular expansion and abnormal ventricular shape are seen at E10.5
|
|
|
• ventricular septal defects
|
homeostasis/metabolism
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Ripply3tm1Sjt mutation
(0 available);
any
Ripply3 mutation
(9 available)
Tbx1tm1Bem mutation
(1 available);
any
Tbx1 mutation
(34 available)
|
|
|
embryo
|
|
• absent in the caudal branchial region
|
cardiovascular system
|
|
• at E18.5 the phenotype is identical to mice homozygous null for Tbx1 alone
|
Allelic
Composition |
Ripply3tm1Sjt/Ripply3+
Tbx1tm1Bem/Tbx1+
|
|
Genetic
Background |
involves: 129/Sv * 129S1/Sv * C57BL/6J * SJL |
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Ripply3tm1Sjt mutation
(0 available);
any
Ripply3 mutation
(9 available)
Tbx1tm1Bem mutation
(1 available);
any
Tbx1 mutation
(34 available)
|
|
|
embryo
|
|
• hypotrophic fourth arch at E10.5
|
craniofacial
|
|
• hypotrophic fourth arch at E10.5
|



 Analysis Tools
Analysis Tools