Phenotypes associated with this allele
nervous system
|
|
• YFP+ neurons exhibit one or more grossly enlarged, rounded mitochondria in the perinuclear region of the soma and in proximal segment of dendrites with some cells containing fragmented mitochondria and small, spherical mitochondrial clustered around the nucleus
• YFP+ neurons exhibit a progressive reduction in distal axonal mitochondrial numbers compared with control cells
• however, mitochondria in the distal dopamine axons are morphologically spared
|
|
|
• neurons exhibit less intense staining with a retrograde tracer (fluorogold) suggesting impaired retrograde transport and/or uptake of the tracer compared with control cells
|
nervous system
|
|
• triple mutants show same phenotype as Tfamtm1Lrsn, Slc6a3tm1(cre)Lrsn mice; neuronal inclusions remain indicating that additional lack of Snca is not required for develoment of the Parkonsonian phenotype
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Tfamtm1Lrsn mutation
(1 available);
any
Tfam mutation
(11 available)
Tg(Ckmm-cre)1Lrsn mutation
(0 available)
|
|
|
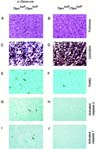
Heart histology of Tfamtm1Lrsn/Tfamtm1Lrsn Tg(Ckmm-cre)1Lrsn/0 mice
mortality/aging
|
|
• die at 2-4 weeks of age
|
growth/size/body
|
|
• cessation of weight gain from P10 onwards
|
cardiovascular system
|
|
• ECG changes under isofluorane anesthesia
|
|
|
• decrease in peak aortic blood flow velocity under isofluorane anesthesia
|
|
|
• mutants exhibit a significant increase in apoptosis of cardiomyocytes
• however, no evidence of fibrosis, necrosis, or inflammatory cell infiltration is seen in the hearts
|
homeostasis/metabolism
behavior/neurological
|
|
• decreased spontaneous movement from P10 onwards
|
muscle
|
|
• mutants exhibit a significant increase in apoptosis of cardiomyocytes
• however, no evidence of fibrosis, necrosis, or inflammatory cell infiltration is seen in the hearts
|
cellular
|
|
• mutants exhibit a significant increase in apoptosis of cardiomyocytes
• however, no evidence of fibrosis, necrosis, or inflammatory cell infiltration is seen in the hearts
|
|
|
• mosaic respiratory chain deficiency in the myocardium
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Cnptm1(cre)Kan mutation
(0 available);
any
Cnp mutation
(26 available)
Tfamtm1Lrsn mutation
(1 available);
any
Tfam mutation
(11 available)
|
|
|
mortality/aging
|
|
• most mice die by 12 weeks of age
|
nervous system
|
|
• in the proximal and distal small intestine at 2 and 7 weeks
• however, the number of enteric neurons in the colon is not significantly different
|
|
|
• in the proximal and distal small intestine at 2 and 7 weeks
• however, the number of glia in the colon is not significantly different
|
|
|
• loss of nitrergic inhibitory neuron
|
|
|
• profound in more proximal regions of the small intestine
|
|
|
• of enteric neurons in proximal and distal small intestine
|
growth/size/body
|
|
• at about 6 to 8 weeks of age
|
digestive/alimentary system
|
|
• massive dilation within the proximal small bowel with dark-colored luminal content and relative contraction of the distal small bowel with no stool pellets in the colon or rectum
• small diameter small intestine
• however, no stenosis or mechanical cause of the obstruction is evident
|
cellular
|
|
• in enteric neurons and glia
|

Mitochondria abnormalities in Tfamtm1Lrsn/Tfamtm1Lrsn Slc6a3tm1(cre)Lrsn/Slc6a3+ Gt(ROSA)26Sortm1Lrsn/Gt(ROSA)26Sor+ neurons
nervous system
|
|
• YFP+ neurons exhibit one or more grossly enlarged, rounded mitochondria in the perinuclear region of the soma and in proximal segment of dendrites with some cells containing fragmented mitochondria and small, spherical mitochondrial clustered around the nucleus
• YFP+ neurons exhibit a progressive reduction in distal axonal mitochondrial numbers compared with control cells
|
cellular
|
|
• smaller mitochondrial and the largest aggregates in a rare number of neurons exhibit reduced import competence compared with mitochondria in control cells
|
behavior/neurological
|
|
• impaired from 20 weeks of age
|
growth/size/body
muscle
|
|
• rigidity from 20 weeks of age
|
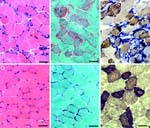
Skeletal muscle analysis of Tfamtm1Lrsn/Tfamtm1Lrsn Myl1tm1(cre)Sjb/Myl1+ mice
mortality/aging
|
|
• death occurs at 4-5 months of age
|
muscle
|
|
• increased muscle fiber size containing enlarged mitochondria with distorted cristae
|
|
|
• shorter contraction times and shorter twitch-relaxation times
• decreased absolute muscle force
|
cellular
|
|
• respiratory chain dysfunction in skeletal muscle
|
behavior/neurological
growth/size/body
|
|
• beginning at 4-5 months of age
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Tfamtm1Lrsn mutation
(1 available);
any
Tfam mutation
(11 available)
Tg(Camk2a-cre)1Lfr mutation
(2 available)
|
|
|
mortality/aging
|
|
• at 5-6 months of age, preceded by 1-2 weeks of rapid physical deterioration
|
nervous system
|
|
• in corpus callosum, hippocampus; progressive with age
|
|
|
• degeneration of cortical organization in neocortex
|
|
|
• neuronal apoptosis in neocortex and hippocampus; progressive with age
|
cellular
|
|
• resipratory chain dysfunction in neurons of the neocortex and hippocampus
|
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Tfamtm1Lrsn mutation
(1 available);
any
Tfam mutation
(11 available)
Tg(Ins2-cre)25Mgn mutation
(2 available)
|
|
|
homeostasis/metabolism
endocrine/exocrine glands
|
|
• normal numbers at 7 weeks, absent at 37 weeks
|
cellular
|
|
• mitochondrial DNA depletion
|
|
|
• respiratory chain dysfunction in pancreatic islets
|
mortality/aging
|
|
• mutants have to be terminated at ~45 weeks due to poor general condition
|
behavior/neurological
|
|
• mice aged 14-15 weeks display decreased exploratory activity
|
|
|
• progressive Parkinsonian symptoms are observed, with tremor observed at 20 weeks of age
|
|
|
• apparent limb rigidity is observed at 20 weeks of age
|
nervous system
|
|
• majority of neurons contain small cytoplasmic aggregates, detected at 6 weeks through 43 weeks
• inclusions are present in most dopaminergic midbrain neurons and the mean size increased as the neurodegeneration progressed
• large, partially electron-dense bodies located in dendritic structures close to neuronal somata are observed at 11 weeks; some of these bodies have an amorphous content with a diffuse lining, while others display tubular formations and have distinct double layer membranes which are ultrastructurally typical of mitochondrial membranes
|
|
|
• dopaminergic (DA) neuron loss is observed in the dorsolateral striatum at 12 weeks of age, progressing to involve most of the dorsal and ventral striatum with age
• tyrosine hydroxylase-expressing midbrain neurons show a slow progressive cell loss, which is more marked and starts sooner in the substantia nigra compared to the ventral tegmental area
|
muscle
|
|
• twitching is observed at 20 weeks of age
|
homeostasis/metabolism
|
|
| Find Mice |
Using the International Mouse Strain Resource (IMSR)
Mouse lines carrying:
Tfamtm1Lrsn mutation
(1 available);
any
Tfam mutation
(11 available)
Tg(Myhca-cre)1Lrsn mutation
(0 available)
|
|
|
mortality/aging
|
|
• animals surviving past neonatal stage survive for several months; variable penetrance speculated to be the result of a modifying gene
|
|
|
• 75% of animals die as neonates
|
cardiovascular system
cellular
|
|
• respiratory chain dysfunction in heart; variable penetrance speculated to be the result of a modifying gene
|
growth/size/body



 Analysis Tools
Analysis Tools